HEALTHCARE COST AND UTLIZATION PROJECT – HCUP
A FEDERAL-STATE-INDUSTRY PARTNERSHIP IN HEALTH DATA
Sponsored by the Agency for Healthcare Research and Quality
INTRODUCTION TO
THE HCUP NATIONWIDE READMISSIONS DATABASE (NRD)
2013
| These pages provide introductory-level information about the NRD. For full documentation and notification of changes, visit the HCUP User Support (HCUP-US) website at http://www.hcup-us.ahrq.gov. |
November 2015
Agency for Healthcare Research and Quality
Healthcare Cost and Utilization Project (HCUP)
Phone: (866) 290-HCUP (4287)
E-mail: hcup@ahrq.gov
website: http://www.hcup-us.ahrq.gov
NRD Data and Documentation Distributed by:
HCUP Central Distributor
Phone: (866) 556-4287 (toll-free)
Fax: (866) 792-5313
E-mail: HCUPDistributor@ahrq.gov
Table of Contents
Skip Table of Contents
HCUP NATIONWIDE READMISSIONS DATABASE (NRD) SUMMARY OF DATA USE LIMITATIONS |
|
***** REMINDER ***** |
All users of the NRD must take the on-line HCUP Data Use Agreement (DUA) training course, and read and sign a Data Use Agreement. † Authorized users of HCUP data agree to the following restrictions: ‡
Any violation of the limitations in the Data Use Agreement is punishable under Federal law by a fine of up to $10,000 and up to 5 years in prison. Violations may also be subject to penalties under State statutes. |
† The on-line Data Use Agreement training session and the Data Use Agreement are available on the HCUP User Support (HCUP-US) website at http://www.hcup-us.ahrq.gov. |
HCUP CONTACT INFORMATION
All HCUP data users, including data purchasers and collaborators, must complete the online HCUP Data Use Agreement (DUA) Training Tool, and read and sign the HCUP Data Use Agreement. Proof of training completion and signed Data Use Agreements must be submitted to the HCUP Central Distributor as described below.
The on-line DUA training course is available at: http://www.hcup-us.ahrq.gov/tech_assist/dua.jsp.
The HCUP Nationwide Data Use Agreement is available on the AHRQ-sponsored HCUP User Support (HCUP-US) website at: http://www.hcup-us.ahrq.gov
HCUP Central Distributor
Data purchasers will be required to provide their DUA training completion code and will execute their DUAs electronically as a part of the online ordering process. The DUAs and training certificates for collaborators and others with access to HCUP data should be submitted directly to the HCUP Central Distributor using the contact information below.
The HCUP Central Distributor can also help with questions concerning HCUP database purchases, your current order, training certificate codes, or invoices, if your questions are not covered in the Purchasing FAQs on the HCUP Central Distributor website.
HCUP User Support:
Information about the content of the HCUP databases is available on the HCUP User Support (HCUP-US) website (http://www.hcup-us.ahrq.gov). If you have questions about using the HCUP databases, software tools, supplemental files, and other HCUP products, please review the HCUP Frequently Asked Questions or contact HCUP User Support:
|
WHAT IS THE NATIONWIDE READMISSIONS DATABASE (NRD)?
|
|
|
UNDERSTANDING THE NRD
|
|
HEALTHCARE COST AND UTILIZATION PROJECT — HCUP
A FEDERAL-STATE-INDUSTRY PARTNERSHIP IN HEALTH DATA
Sponsored by the Agency for Healthcare Research and Quality
HCUP NATIONWIDE READMISSIONS DATABASE (NRD)
ABSTRACT
The Nationwide Readmissions Database (NRD) is part of the Healthcare Cost and Utilization Project (HCUP) that is sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NRD addresses a large gap in healthcare data - the lack of nationally representative information on hospital readmissions for all types of payers and the uninsured. The NRD was created to enable analyses of national readmission rates and to support public health professionals, administrators, policymakers, and clinicians in their decision making.
The NRD is drawn from HCUP State Inpatient Databases (SID) that contain reliable, verified patient linkage numbers that can be used to track a person across hospitals within a State, while adhering to strict privacy guidelines. The 2013 NRD is constructed from 21 SID. These States are geographically dispersed and account for 49.3 percent of the total U.S. resident population and 49.1 percent of all U.S. hospitalizations. See Appendix A, Table 1 for a list of data organizations participating in the NRD.
The NRD includes community hospitals, excluding rehabilitation or long-term acute care hospitals. All discharges from the SID are included except the following:
After exclusions, the 2013 NRD contains about 85 percent of SID discharges from the participating states. Unweighted, the NRD contains approximately 14 million discharges each year. Weighted, it estimates roughly 36 million discharges in the United States.
The NRD is designed to be flexible to various types of analyses of national readmissions for all types of payers and the uninsured. The criteria to determine the relationship between multiple hospital admissions for an individual patient is left to the analyst using the NRD. Outcomes of interest include national readmission rates, reasons for returning to the hospital for care, and the hospital costs for discharges with and without readmissions. The NRD is not designed to support regional, State-, or hospital-specific readmission analyses.
Access to the NRD is open to users who sign a Nationwide Databases Data Use Agreement (DUA). Uses are limited to research and aggregate statistical reporting. For more information on the NRD, visit the AHRQ-sponsored HCUP User Support (HCUP-US) website at http://www.hcup-us.ahrq.gov.
INTRODUCTION TO THE NATIONWIDE READMISSIONS DATABASE (NRD)
Overview of NRD Data
The Healthcare Cost and Utilization Project (HCUP) Nationwide Readmissions Database (NRD) was created to enable analyses of national readmission rates and to support public health professionals, administrators, policymakers, and clinicians in their decision making. Reducing hospital readmission rates is a key strategy for improving the quality of healthcare while containing cost. The NRD is designed to be flexible to various types of readmission analyses. The database includes discharges for patients with and without repeat hospital visits in a year and those who have died in the hospital. Repeat visits may or may not be related. The criteria to determine the relationship between hospital admissions is left to the analyst using the NRD.
States Included in the NRD
In their SID, some HCUP Partner organizations provided synthetic patient linkage numbers that can be used to track patients within and across hospitals in a particular State. Unique combinations of the patient linkage number and the patient's date of birth and sex constituted a verified patient linkage number.
States selected for the NRD have verified patient linkage numbers on at least 90 percent of adult discharges. From these States, the NRD included discharges from patients aged 1 year and older. Discharges aged 0 were only retained from States that had verified patient linkage numbers on more than 90 percent of discharges aged 0. Appendix A, Table 1 identifies the statewide data organizations that contribute to the NRD. Appendix A, Figure 1 displays the geographic distribution of the 21 HCUP Partner organizations participating in the 2013 NRD. Based on U.S. Census Bureau data, the 2013 NRD accounts for 49.3 percent of the U.S. population and 49.1 percent of U.S. hospitalizations reported in the American Hospital Association (AHA) Annual Survey Database.
Hospitals Included in the NRD
The SID contains inpatient discharges for all hospitals provided by the HCUP Partners (e.g., community, specialty, and Federal hospitals). The American Hospital Association (AHA) defines community hospitals as "all non-Federal, short-term, general, and other specialty hospitals, excluding hospital units of institutions." Specialty hospitals included in the AHA definition of community hospitals are obstetrics-gynecology, ear-nose-throat, short-term rehabilitation, orthopedic, pediatric institutions, and long-term acute care (LTAC) facilities. Also included are public hospitals and academic medical centers.
The NRD is limited to data from community hospitals that are not rehabilitation or LTAC facilities. Noncommunity hospitals were excluded because of inconsistent capture of data across HCUP States. We excluded rehabilitation or LTAC hospitals because they treat a unique patient population that has longer stays and higher costs. Information on the percentage of SID discharges excluded by type of exclusion is provided in Appendix A, Table 2. Details on the number of hospitals in the NRD are provided in Appendix A, Table 3.
Discharges Included in the NRD
All SID discharges from selected States and hospitals were included, with a few exceptions. Records with missing or unverified patient linkage numbers were excluded from the NRD. All discharges aged 0 from SID records with verified patient linkage numbers on less than 90 percent of discharges were excluded.
Another concern was verified patient linkage numbers that did not appear to track an individual within the year for the following reasons:
Discharges for patient linkage numbers considered questionable because of these three criteria were excluded from the NRD.
Discharge-specific exclusions such as the removal of unverified, missing, or questionable patient linkage numbers impacted individual hospitals if they had more than 50 percent of their annual discharges excluded. These hospitals were not good candidates for a readmission analysis because too many of their discharges could not be tracked over time, and were therefore excluded from the NRD. Information on the percentage of SID discharges excluded by type of exclusion is provided in Appendix A, Table 2.
There were no exclusions for certain types of patients or clinical conditions. Therefore, we included conditions such as childbirth that generally do not result in a readmission. We retained discharges that resulted in an in-hospital death because these were possible readmission records. We also included discharges for residents and nonresidents of the State in which they were treated. Although most patients seek treatment at hospitals in their State of residence, there are occasions when patients are treated at hospitals in another State. Hospitals that specialize in a certain types of care may attract patients from across the United States. In addition, hospitals near State borders frequently treat patients that reside in neighboring States. The data element RESIDENT identifies a discharge as a resident of the State in which he or she received hospital care. Details on the number of discharges in the NRD are provided in Appendix A, Table 3.
Hospital administrative databases like the NRD and SID are "discharge-level" files, meaning that each record represents one discharge abstract from an inpatient stay. If a patient visits the hospital multiple times in a given year, the SID includes separate records for each inpatient stay. In addition, if a patient is transferred between hospitals within the State, the SID contain two discharge records, one record from the first hospitalization and a second record from the latter hospitalization.1 Readmission analyses do not usually allow the hospitalization at the receiving hospital to be counted as a readmission. To eliminate this possibility, we collapsed the pairs of records representing a transfer into a single "combined" record in the NRD and removed the original separate discharge records from the NRD. We defined transfer records as having all of the following characteristics:
We defined a discharge as a same-day stay if the discharge date for one inpatient stay was the same as the admission date of a second stay for the same patient (same as transfers), but there was no indication of a transfer by the discharge disposition or admission source. Same-day stays may or may not have involved different hospitals. Same-day stays may indicate that a patient was discharged too soon and then needed to be returned to the hospital on the same day. However, it was also possible that these were transfer records with an incorrect or missing discharge disposition and admission source. A more detailed description of the methodology used to identify and combine transfer and same-day stays is provided in Appendix B.
Summary of Hospitals and Discharges Included in the NRD
The NRD includes community hospitals and excludes the following types of hospitals:
All discharges from included hospitals were retained in the NRD with the following exceptions:
Information on the percentage of SID discharges excluded by type of exclusion is provided in Appendix A, Table 2. There were no exclusions for certain types of patients or clinical conditions. Discharges that resulted in an in-hospital death were included because these were possible readmission records. Discharges for residents and nonresidents of the State in which they were treated were also included. In addition, pairs of discharge records representing transfers (i.e., one discharge record from the sending hospital and one discharge record from the receiving hospital) were collapsed into a single record so that the hospitalization at the receiving hospital could not be counted as a readmission. Details on the number of States, hospitals, and discharges in the NRD are provided in Appendix A, Table 3.
State-Specific Restrictions
Some sources that contributed data to the NRD imposed restrictions on the release of certain data elements or discharges that could be included in the database. In addition, because of confidentiality laws, some data sources were prohibited from providing HCUP with discharge records that indicated specific medical conditions, such as HIV/AIDS or behavioral health. Detailed information on these State-specific restrictions is available in Appendix C.
File Structure of the NRD
The NRD includes three discharge-level files and one hospital-level file:
NRD Data Elements
The coding of data elements in the NRD is consistent with other HCUP databases. The following three objectives guided the definition of data elements in all HCUP databases:
More information on the coding of HCUP data elements is available on the HCUP User Support (HCUP-US) website (http://www.hcup-us.ahrq.gov/db/coding.jsp).
The NRD contains more than 100 clinical and non-clinical variables provided in a hospital discharge abstract, such as:
Appendix D identifies the data elements in each NRD file:
The tables in Appendix D provide summary documentation for the data. Please refer to the NRD documentation located on the HCUP-US website (http://www.hcup-us.ahrq.gov) for comprehensive information about data elements and the files.
Getting Started
The NRD is an extremely large database that requires sophisticated statistical software for analysis. The following computer properties are needed in order to load and analyze the NRD data:
The total size of the comma-separated version (CSV) of the NRD is 10 GB. The NRD files loaded into SAS are about 12 GB. In SAS, the largest use of space typically occurs during a sort, which requires work space about three times the size of the file. Thus, the NRD files would require at least 36 GB of available workspace to perform a sort. Most SAS data steps will require twice the storage of the file, so that both the input and output files can coexist. The NRD files loaded into SPSS are under 23 GB. Because Stata loads the entire file into memory, it may not be possible to load every data element in the NRD Core file into Stata. Stata users will need to maximize memory and use the "_skip" option to select a subset of variables. More details are provided in the Stata load programs.
With a file of this size and without careful planning, space could easily become a problem in a multi-step program. It is not unusual to have several versions of a file marking different steps while preparing it for analysis, and there may be more versions for the actual analyses. Therefore, the amount of space required could escalate rapidly.
Copying and Decompressing the Comma-Delimitated Files
The NRD is distributed as CSV files compressed with SecureZIP® from PKWARE. To copy and decompress the NRD files from the DVD, the following steps are outlined:
Downloading and Running the Load Programs
Programs to load the data into SAS, SPSS, or Stata, are available on the HCUP User Support website (HCUP-US). These steps are used to download and run the load programs:
NRD Documentation
Comprehensive documentation for the NRD files is available on the HCUP-US website (http://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp). Users of the NRD can access complete file documentation, including variable notes, file layouts, and summary statistics. Similarly, data users can download SAS, SPSS, and Stata load programs. These important resources help the user understand the structure and content of the NRD and aid in using the database.
To locate the NRD documentation on HCUP-US, the user is instructed to:
Appendix A, Table 4 details the comprehensive NRD documentation available on HCUP-US.
HCUP Online Tutorials
For additional assistance, AHRQ has created the HCUP Online Tutorial Series, a series of free, interactive courses that provide training on technical methods for conducting research with HCUP data. Topics include an HCUP Overview Course and these tutorials that are helpful to NRD data users:
Other tutorials about the design or use of other HCUP databases also are available. The Online Tutorial Series is located on the HCUP-US website at http://hcup-us.ahrq.gov/tech_assist/tutorials.jsp.
HCUP Methods Series Reports on Readmission Methodology
HCUP has created two Methods Series reports that provide additional information readmissions:
SAMPLING DESIGN OF THE NRD
The NRD was built to facilitate analyses of both all-cause and condition-specific readmissions. National estimates can be created by applying weighting and stratification methods.
Target Universe
The target universe was limited to inpatient discharges treated at community hospitals in the United States that were not rehabilitation or LTAC facilities. Information on the target universe was available from the American Hospital Association (AHA) Annual Survey of Hospitals. The AHA Survey includes information on the number of inpatient discharges and hospital characteristics such as ownership, number of beds, and location
Sampling Frame
The sampling frame for the NRD was limited to discharges for patients treated at community hospitals in the NRD States that were not rehabilitation or LTAC facilities. All of the discharges in the sampling frame were included making the NRD a sample of convenience. Sampling discharges or hospitals was not recommended because the sample needed to balance the database's ability to estimate readmissions for common conditions such as chronic illnesses with the ability to estimate readmissions for rare diseases such as sickle cell anemia. Developing the database using a 100 percent sample allows researchers to study both all-cause and condition-specific readmissions.
Discharge Weights
This section explains the need for post-stratification for weighting the sampling frame to the target universe and the weighting strategy. We use the term post-stratification because the stratification was performed after sampling. Discharge weights for national estimates were developed using the target universe as the standard. Although we calculated discharge-level weights for the NRD, we did not calculate hospital-level weights. The NRD is not designed to support hospital-specific analyses. The NRD was a 100 percent sample of discharges, not hospitals; hospital weights were not applicable.
Post-Stratification for Weighting
Post-stratification for the purpose of weighting allowed us to compensate for any over- or under-represented types of hospitals and discharges in the sampling frame (the NRD) with respect to the distribution in the target universe (AHA data). The NRD was post-stratified by hospital and patient characteristics. We knew from the National (Nationwide) Inpatient Sample (NIS) design that the following hospital characteristics explained significant differences in inpatient outcomes: census region, urban/rural location, hospital teaching status, size of the hospital defined by the number of beds, and hospital control.5
We had excluded discharges with unverified and missing patient linkage numbers because these patients cannot be tracked across time. In an examination of the distribution of patient age and sex between discharges with verified and unverified/missing patient linkage numbers, we determined that the majority of the unverified discharges (52.7 percent) were females aged 18-44 years old. In addition, we compared 30-day all-cause readmission rates in 2011 by age-sex categories across States and saw variation between SID with a high percentage of verified patient linkage numbers and States with a lower percentage of verified patient linkage numbers. These analyses demonstrated that there were differences between discharges with and without patient linkage numbers by sex and age. Therefore, the NRD was also post-stratified by sex and five age groups (0, 1-17, 18-44, 45-64, and 65 and older).
Weighting
We based the discharge counts by stratum for the target universe totals on all SID discharges from all HCUP Partners, unless there were missing hospitals. If there were hospitals missing from the stratum according to the AHA, then the target universe total included SID discharges for all available hospitals plus the AHA discharge counts for the missing hospitals. This approach was consistent with the NIS and took advantage of the fact that the SID included over 95 percent of discharges from community hospitals that are not rehabilitation or LTAC hospitals in the United States. Discharge counts for the sampling frame were based on the NRD discharges (after the exclusion of States, hospitals, and discharges). To determine discharge-level weights, we summarized the number of discharges for the target universe and sampling frame by stratum defined by hospital characteristics (census region, urban/rural location, hospital teaching status, size of the hospital defined by the number of beds, and hospital control) and patient characteristics (sex and five age groups [0, 1-17, 18-44, 45-64, and 65 and older]). Within each stratum, s, each NRD inpatient admission received a weight:
DISCWTi,j = Ns(universe)i,j ÷ Ns(sample)i,j
where Ns(universe)i,j represents the number of inpatient admissions at community hospitals that were not a rehabilitation or LTAC hospital in the universe within stratum s for sex i and age group j; Ns(sample)i,j is the number of inpatient admissions in the sampling frame for sex i and age group j. Age group j included ages 0, 1-17, 18-44, 45-64, 65 and older. Therefore, each discharge's weight (DISCWTi,j) is equal to the number of inpatient admissions it represents in stratum s for sex i and age group j during that year.
To improve reliability of the age distribution of the SID discharges, we collapsed the strata prior to the weight calculations such that we included at least two SID hospitals and at least 100 discharges from the SID in each stratum. In addition, we collapsed the strata to include at least two hospitals in the sample frame. We first collapsed the stratum across control/ownership, combining either the two private designations or all three types of control (public, private not-for-profit, and private for-profit). If the stratum combined across control still lacked a sufficient number of hospitals or discharges, then the location category was collapsed. Small and large metropolitan areas are combined or micropolitan and rural areas combined. Lastly, if the stratum still lacked a sufficient number of hospitals or discharges, then the bed-size category was collapsed with large and medium hospitals combined. There was no collapsing of strata across region or teaching status. In addition, we adjusted weights if a hospital was missing data for one or more quarters in the year. The range of weights by age and sex are provided in Appendix A, Table 5.
Final NRD Sample
The NRD is an annual file constructed using one calendar year of discharge data. Included discharges were treated at community hospitals (excluding rehabilitation or LTAC hospitals) for which the majority of their discharges had patient linkage numbers that were verified and not questionable. Discharge weights were calculated using post-stratification on hospital characteristics (census region, urban-rural location, teaching status, bed size, and hospital control) and patient characteristics (sex and five age groups [0, 1-17, 18-44, 45-64, and 65 and older]). The target universe of inpatient discharges in the United States was estimated for each stratum using SID total discharges augmented by AHA discharge counts when hospitals were not reported in the SID. Details on the number of States, hospitals, and discharges in the NRD are provided in Appendix A, Table 3. The range of discharge weights by age and sex are provided in Appendix A, Table 5. The NRD is designed to be flexible to various types of analyses of national readmissions. The NRD is not designed to support regional, State- or hospital-specific readmission analyses.
Limitations of the NRD
The NRD contains over 14 million inpatient discharge records and over 100 clinical and non-clinical data elements. A multitude of research studies can be conducted with the data, but there are some limitations.
HOW TO USE THE NRD FOR READMISSION ANALYSES
This section provides a brief synopsis of special considerations for using the NRD. For more details, refer to the comprehensive documentation on the HCUP-US website (http://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp).
All persons using the NRD (whether or not they are the original recipient of the data) must complete the online Data Use Agreement Training Tool available on the HCUP-US website (http://www.hcup-us.ahrq.gov/tech_assist/dua.jsp) and then read and sign a Data Use Agreement. A copy of the signed Data Use Agreements must be sent to the HCUP Central Distributor. See page 2 of this document for the mailing address.
NRD Data Elements Critical to Tracking a Patient and Determining the Time Between Admissions
For any readmission analysis of inpatient stays, three HCUP data elements are critical to tracking a patient and determining the time between admissions: NRD_VisitLink, NRD_DaysToEvent, and LOS (length of stay).
Patient Linkage Number (NRD_VisitLink)
NRD_VisitLink is the linkage variable for all inpatient stays associated with a unique patient. All discharges in the NRD include a value for NRD_VisitLink. The value was assigned based on a unique combination of the synthetic patient linkage number provided by the HCUP Partner, date of birth, and sex. No verified patient linkage number was assigned if any one of the three pieces of information was missing. Because of discharge-level exclusions, NRD_VisitLink is always coded on records in the NRD.
Although the term patient linkage number is used to describe the information in the NRD data element NRD_VisitLink, the values are not recognizable as specific patient information. NRD_VisitLink does not include the values of the encrypted person's social security number, date of birth, or sex.
Time Between Admissions (NRD_DaysToEvent and LOS)
NRD_DaysToEvent is the number of days from a randomly chosen "start date" to the admission date for each patient's discharge. The actual admission and discharge dates could not be included on the NRD because they were considered highly sensitive information according to Health Insurance Portability and Accountability Act (HIPAA) guidelines. The coding scheme for NRD_DaysToEvent was designed to adhere to these strict privacy guidelines and protect patient confidentiality.
Each verified patient linkage number (NRD_VisitLink) was assigned a unique start date that was used to calculate NRD_DaysToEvent for all visits associated with that NRD_VisitLink value. The variable NRD_DaysToEvent was the difference between the visit's admission date and the start date associated with the NRD_VisitLink. NRD_DaysToEvent was reported as missing if the admission date was unavailable.
For readmission analyses, determining the number of days between the end of one admission and the start of the next admission is critical. We did not include any single data element specific to this timing difference in the NRD because the calculation is dependent on which two discharges are of interest for the readmission study. For example, a study of readmissions for diabetes might only consider the number of days between two diabetes discharges, whereas a study of post-surgery infections might consider any discharge in 30 days.
Because NRD_DaysToEvent was based on the admission date, the calculation of days needs to be the difference of NRD_DaysToEvent between two selected discharges for a unique verified patient linkage number (NRD_VisitLink), adjusted for the length of stay. Consider the following example:
If NRD_DaysToEvent or LOS was missing, then it was not possible to determine the number of days to a subsequent admission. We considered removing the discharges with missing NRD_DaysToEvent and LOS from the NRD, but these data elements were very rarely missing. LOS was only critical if it was missing on the first admission in a series. If the admission was the second in the series, then LOS was not pertinent.
The lowest value of NRD_DaysToEvent is the earliest inpatient stay in the year for a patient. It is important to remember that if patient A has a value of 605 for NRD_DaysToEvent and patient B has a value of 300 for NRD_DaysToEvent, patient B's hospital stay did not necessarily take place prior to patient A's stay. In fact, Patient B's NRD_DaysToEvent value has no relation to Patient A's NRD_DaysToEvent value. Because of the use of a random start date in the calculation of NRD_DaysToEvent, the value of NRD_DaysToEvent cannot be compared across patients.
Additional information about the HCUP revisit variables is available on the HCUP User Support website (http://www.hcup-us.ahrq.gov/toolssoftware/revisit/revisit.jsp).
Defining Readmissions
The NRD was designed to support many different types of readmission analyses. Analysts can use the information contained in the NRD to define the index event and readmission specific to their topic of interest. Common terminology is first defined:
The NRD was designed to support many different types of readmission analyses. Analysts can use the information contained in the NRD to define the index event and readmission specific to their topic of interest. We discuss the following analytic considerations for defining index events and readmissions:
Defining the Index Event
The index event is typically defined by a combination of clinical criteria. Inclusion and exclusion criteria should be used to define an index event indicator that identifies NRD discharges as an index event specific to the analysis of interest. The NRD did not include a variable for index events because they are specific to each analysis. The NRD included the information necessary to define different types of index events.
Criteria can include, but are not limited to, age of the patient and specific diagnoses and/or procedures. The NRD contained various data elements that can be used for inclusion criteria:
Possible exclusion criteria include the following:
The annual NRD files include inpatient stays that were discharged in that data year. For example, the 2013 NRD includes admissions that began in 2012 and were discharged in 2013. In contrast, we did not include admissions that began later in 2013 and extended into 2014 in the 2013 NRD. About one percent of discharges in the NRD started in the previous year; therefore, we expected that we were missing about one percent of admissions that started in 2013 and were subsequently discharged in 2014. This will be true for each year of the NRD.
Deciding which months should be excluded when qualifying an index event depends on the time that will be allowed for a readmission. For example, if studying 30-day readmissions, the index event might be limited to those occurring in the discharge months of January through November. That allows the month of December for 30 days of follow-up. Although it would be advantageous to be able to select a more specific date for a cut-off, patient confidentially concerns limited the available information on the admission and discharge dates to discharge month (data element DMONTH) and discharge year (data element YEAR).
Specifying the Criteria for a Readmission
Readmission analyses tend to consider one of the following: any subsequent admission regardless of cause, any subsequent admission that does not involve trauma, or any subsequent admission only if the event is "related" to the index event. In addition, a study may consider all readmissions within a time period or just the first readmission. The selection of criteria can dramatically change results. More information on how the results can change is available in an HCUP Method Series report on Methodological Issues when Studying Readmissions and Revisits Using Hospital Administrative Data.9
The NRD includes a number of different diagnosis and procedure-related variables that can be used to examine why a patient returned for hospital care. The NRD does not identify any discharge as a readmission; instead, we include the information necessary to select the appropriate readmission discharges in the NRD. Inclusion and exclusion criteria should be used to define a readmission indicator that identifies NRD discharges as readmissions specific to the analysis of interest.
Selecting the Time Period for Revisits
When determining an appropriate time period for the readmission, considerations include selecting a time that encompasses the same risk of exposure to all patients, seasonality of the disease, and possible external factors. Shorter time frames (7 or 14 days) are often used to make events attributable to hospital acute care; longer time frames may reflect differences in ambulatory care and/or coordination of care.
Reporting Rates of Readmission
Although the definition of readmission rate—number of readmissions divided by number of cases followed— seems simple, our research into readmission rates showed no standard definition. In some cases, the unit of observation was a patient; in others, the unit of observation was index events, and individual patients were counted more than once. Some studies focused on the first readmission following an index event, whereas others counted all readmissions. The definitions of the readmission rate were specific to the purpose of the analyses.
Severity or risk adjustment may also be beneficial when comparing readmission rates across geographical regions, hospital types, or different patient populations. A simple risk adjustment would include the age and sex of the patient. A more complex adjustment might also include comorbidites, severity classified by the 3M All-Patient Refined DRG severity score, patient income quartile, or any other factor that could considerably increase or decrease the risk of subsequent hospital care. The NRD included variables to support these types of analyses in the Core, Severity, and Diagnosis and Procedure Groups files.
Calculating Nationally Weighted Estimates
An analyst using the NRD must use the discharge-level weight DISCWT to produce national estimates. Weighted statistics estimate discharges treated at community hospitals (excluding rehabilitation and LTAC facilities) in the United States.
Variance Calculations
It may be important for researchers to calculate a measure of precision for national estimates based on the NRD. Variance estimates must take into account both the sampling design and the form of the statistic. It is important to understand that the NRD is a sample of convenience from the SID and not a sample of hospitals or discharges. Standard error calculations should take into account the stratified sample (data element NRD_STRATUM) and hospitals defining the clusters (data element HOSP_NRD). One resource for understanding the issues surrounding variance calculations for the NRD is the HCUP Methods Series report #2010-05 Inferences with HCUP State Databases Final Report on the HCUP-US website (http://www.hcup-us.ahrq.gov/).
To accurately calculate variances from the NRD, appropriate statistical software and techniques must be used. A multitude of statistics can be estimated from the NRD data. Several computer programs that calculate statistics and their variances from sample survey data are listed in the next section. Some of these programs use general methods of variance calculations (e.g., the jackknife and balanced half-sample replications) that take into account the sampling design. However, it may be desirable to calculate variances using formulas specifically developed for certain statistics.
These variance calculations are based on finite-sample theory, which is an appropriate method for obtaining cross-sectional, nationwide estimates of outcomes. According to finite-sample theory, the intent of the estimation process is to obtain estimates that are precise representations of the nationwide population at a specific point in time. In the context of the NRD, any estimates that attempt to accurately describe readmissions during a specific year should be governed by finite-sample theory. Examples would be estimates of expenditure and utilization patterns.
Computer Software for Weighted and Variance Calculations
The NRD discharge weights are needed to calculate national estimates of readmission counts and rates. In most cases, computer programs are readily available to perform these calculations. Several statistical programming packages allow weighted analyses10 For example, nearly all SAS procedures incorporate weights. In addition, several statistical analysis programs have been developed to specifically calculate statistics and their standard errors from survey data. Version 8 or later of SAS contains procedures (PROC SURVEYMEANS and PROC SURVEYREG) for calculating statistics based on specific sampling designs. Stata and SUDAAN are two other common statistical software packages that perform calculations for numerous statistics arising from the stratified, single-stage cluster sampling design.
NRD READMISSION RATES REPORTED ON THE HCUPNET WEBSITE
Readmission rates generated from the NRD are available on HCUPnet, a free online query system based on data from the HCUP (https://datatools.ahrq.gov/hcupnet). We define in this section how the readmission rates are calculated for HCUPnet to provide an example of how the NRD data elements might be used to define an index event, 30-day readmission, and readmission rates. Other types of readmission analyses are possible with the NRD; this is just one of many possible applications.
For the readmission rates on HCUPnet, we defined an index event as follows:
For example, if a patient was discharged alive with a nonmissing length of stay on January 10, January 20, January 26, and March 30, all four discharges would qualify as index admissions.
For the readmission rates on HCUPnet, we defined readmissions as follows:
On HCUPnet, we defined the readmission rates as the percentage of index admissions that had at least one readmission within 30 days.
Rates were not risk adjusted.
Consider an example of the 30-day, all-cause readmission rate for any diagnosis for a patient discharged alive on January 10, January 20, January 26, and March 30. Each admission is considered an index.
The 30-day readmission rate is 50 percent, because there are two 30-day readmissions for the four index admissions.
HCUPnet can be used to query 30-day readmission rates by the following:
HCUPnet reports readmission counts, rates and costs stratified by the following characteristics of the index stay:
APPENDIX A: NRD INTRODUCTORY INFORMATION
Table A.1. HCUP Partners Participating in the 2013 NRD
| State | HCUP Data Source |
|---|---|
| Arkansas | Arkansas Department of Health |
| California | California Office of Statewide Health Planning and Development |
| Florida | Florida Agency for Health Care Administration |
| Georgia | Georgia Hospital Association |
| Hawaii | Hawaii Health Information Corporation |
| Iowa | Iowa Hospital Association |
| Louisiana | Louisiana Department of Health and Hospitals |
| Massachusetts | Massachusetts Center for Health Information and Analysis |
| Missouri | Missouri Hospital Industry Data Institute |
| Nebraska | Nebraska Hospital Association |
| New Mexico | New Mexico Department of Health |
| Nevada | Nevada Department of Health and Human Services |
| New York | New York State Department of Health |
| South Carolina | South Carolina Revenue and Fiscal Affairs Office |
| South Dakota | South Dakota Association of Healthcare Organizations |
| Tennessee | Tennessee Hospital Association |
| Utah | Utah Department of Health |
| Virginia | Virginia Health Information |
| Vermont | Vermont Association of Hospitals and Health Systems |
| Washington | Washington State Department of Health |
| Wisconsin | Wisconsin Department of Health Services |
Figure A.1. HCUP States Participating in the 2013 NRD
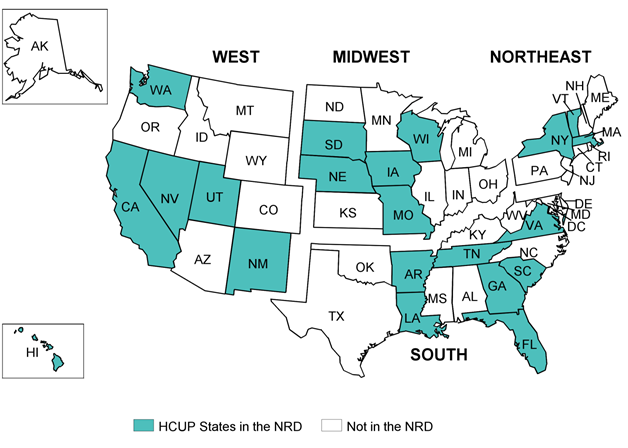
Table A.2. Percentage of SID Discharges in the NRD by Type of Discharge
| Type of Discharge | Percentage of SID Discharges, 2013 |
|---|---|
| Included in the NRD | 84.72 |
| Excluded from the NRD | 15.28 |
| Hospital-level exclusions | |
| Noncommunity hospitals | 2.54 |
| Rehabilitation or LTAC hospitals | 0.27 |
| Discharge-level exclusions | |
| Discharges from patients with an age of 0 (from 12 of 21 SID) | 7.56 |
| Discharges with missing or unverified patient linkage numbers | 4.13 |
| Questionable patient linkage numbers: same patient linkage number on 20 or more discharges | 0.18 |
| Questionable patient linkage numbers: patient is hospitalized after discharged dead | 0.02 |
| Questionable patient linkage numbers: overlapping inpatient stays | 0.46 |
| Discharges from hospitals with more than 50 percent of their total discharges excluded for any of the above causes | 0.13 |
Table A.3. Summary of NRD States, Hospitals, and Inpatient Stays
| Year | States | Number of States for Discharges Aged 1 and Older | Number of States for Discharges Aged 0 | Number of Hospitals | Number of Discharges in the NRD, Unweighted | Number of Discharges in the NRD, Weighted |
|---|---|---|---|---|---|---|
| 2013 | AR, CA, FL, GA, HI, IA, LA, MA, MO, NE, NM, NV, NY, SC, SD,TN, UT, VA, VT, WA, WI | 21 | 9 | 2,006 | 14,325,172 | 35,580,348 |
Table A.4. NRD-Related Reports and Database Documentation Available on HCUP-US
Description of the NRD Files
|
HCUP Tools: Labels and Formats
|
Table A.5. Range of Discharge Weights by Patient Age and Sex
| Patient Age and Sex | Discharge Weight, 2013 | ||
|---|---|---|---|
| Minimum | Average Across Stata | Maximum | |
| Males | |||
| Age 0 | 1.07 | 7.53 | 31.90 |
| Age 0-17 | 1.04 | 3.89 | 18.48 |
| Age 18-44 | 1.13 | 2.88 | 11.33 |
| Age 45-64 | 1.10 | 2.83 | 10.58 |
| Age 65 and older | 1.07 | 2.83 | 11.81 |
| Females | |||
| Age 0 | 1.07 | 7.66 | 35.30 |
| Age 0-17 | 1.04 | 3.55 | 11.28 |
| Age 18-44 | 1.06 | 2.67 | 7.53 |
| Age 45-64 | 1.09 | 2.78 | 9.83 |
| Age 65 and older | 1.06 | 2.83 | 11.68 |
APPENDIX B: HANLDING OF TRANSFERS AND SAME-DAY STAYS
Hospital administrative databases like the NRD and SID are "discharge-level" files, meaning that each record represents one discharge abstract from an inpatient stay. If a patient visits the hospital multiple times in a given year, the SID includes separate records for each inpatient stay. In addition, if a patient is transferred between hospitals within the State, the SID contain two discharge records, one record from the first hospitalization and a second record from the latter hospitalization.11
Defining Transfers and Same-Day Events
Readmission analyses do not usually allow the hospitalization at the receiving hospital to be counted as a readmission. To eliminate this possibility, we collapsed the pairs of records representing a transfer into a single "combined" record in the NRD and removed the original separate discharge records from the NRD. We defined transfer records as having all of the following characteristics:
We defined a discharge as a same-day stay if the discharge date for one inpatient stay was the same as the admission date of a second stay for the same patient (same as transfers), but there was no indication of a transfer by the discharge disposition or admission source. Same-day stays may or may not have involved different hospitals. Same-day stays may indicate that a patient was discharged too soon and then needed to be returned to the hospital on the same day. However, it was also possible that these were transfer records with an incorrect or missing discharge disposition and admission source.
We collapsed records that were part of transfers and same-day stays into a single combined record. These combined records account for about three percent of records in the NRD and are identified by the data element SAMEDAYEVENT. The value of SAMEDAYEVENT is defined as follows:
Please note that if a patient had a discharge disposition of transfer and an admission source that also indicated a transfer, but the discharge date of the first stay did not equal the admission date of the second stay (e.g., the patient was admitted the next day because the transfer occurred at night), the two records are not considered a transfer in the NRD.
Creating a Combined Transfer Record
Combining information across transfer and same-day stay records required specific rules for how to handle different types of information on the pairs of records. We first ordered the pairs of records by earliest occurrence in the year. The different scenarios described below detail how we combined different types of information:
APPENDIX C: STATE-SPECIFIC RESTRICTIONS
The table below enumerates the types of restrictions applied to the Nationwide Readmissions Database. Restrictions include the following types:
For each restriction type the data sources are listed alphabetically by State. Only data sources that have restrictions are included. Data sources that do not have restrictions are not included.
Table C.1. State-Specific Restrictions
| Confidentiality of Records |
|---|
Restricted release of patient's age in years to ensure patient confidentiality:
|
| Missing Discharges for Specific Populations of Patients |
The following data sources may be missing discharge records for specific populations of patients:
|
APPENDIX D: NRD DATA ELEMENTS AND CODES
Table D.1. Data Elements in the NRD Core File
| Category | Data Element Name | Description |
|---|---|---|
| Admission/Discharge | AWEEKEND | Admission on weekend: (0) admission on Monday-Friday, (1) admission on Saturday-Sunday |
| DIED | Indicates in-hospital death: (0) did not die during hospitalization, (1) died during hospitalization | |
| DISPUNIFORM | Disposition of patient, uniform coding: (1) routine, (2) transfer to short term hospital, (5) other transfers, including skilled nursing facility, intermediate care, and another type of facility, (6) home healthcare, (7) against medical advice, (20) died in hospital, (99) discharged alive, destination unknown | |
| DQTR | Coded: (1) Jan-Mar, (2) Apr-Jun, (3) Jul-Sep, (4) Oct-Dec | |
| ELECTIVE | Indicates elective admission: (1) elective, (0) nonelective admission | |
| HCUP_ED | Indicator that discharge record includes evidence of emergency department (ED) services: (0) record does not meet any HCUP ED criteria, (1) ED revenue code was on SID record, (2) ED charge reported on SID record, (3) ED CPT procedure code on SID record, (4) other indication of ED services | |
| YEAR | Discharge year | |
| Clinical Information | DRG | DRG in use on discharge date |
| DRG_NoPOA | DRG assignment made without the use of the present on admission flags for the diagnoses | |
| DRGVER | Grouper version in use on discharge date | |
| DX1-DX25 | Diagnoses, principal and secondary (ICD-9-CM) | |
| DXCCS1-DXCCS25 | CCS category for all diagnoses | |
| E_CCS1-E_CCS4 | CCS category for all External cause of injury codes | |
| ECODE1-ECODE4 | External causes of injury codes (ICD-9-CM) | |
| MDC | MDC in use on discharge date | |
| MDC_NoPOA | MDC assignment made without the use of the present on admission flags for the diagnoses | |
| NCHRONIC | Number of chronic conditions | |
| NDX | Number of diagnoses coded | |
| NECODE | Number of external causes of injury codes coded | |
| NPR | Number of procedures coded | |
| ORPROC | Major operating room procedure indicator: (1) major operating room procedure reported on discharge record, (0) no major operating room procedure reported on discharge record | |
| PR1-PR15 | Procedures, principal and secondary (ICD-9-CM) | |
| PRCCS1-PRCCS15 | CCS category for all procedures | |
| NRD Identifiers | HOSP_NRD | NRD hospital identifier specific to the NRD and is not linkable to any other HCUP or external databases |
| KEY_NRD | NRD record identifier specific to the NRD and not linkable to any other HCUP or external databases | |
| Patient Demographics | AGE | Age in years coded 0-90 years; any age greater than 90 was set to 90. Missing age was imputed using other records with the same patient linkage number. Less than 1000 discharges (0.007 percent) had the age imputed. |
| FEMALE | Indicates sex: (0) male, (1) female. Age was imputed for other records with the same patient linkage number of missing. Missing sex was imputed using other records with the same patient linkage number. Less than 10 discharges had the sex imputed. | |
| PAY1 | Expected primary payer, uniform: (1) Medicare, (2) Medicaid, (3) private insurance, (4) self-pay, (5) no charge, (6) other | |
| PL_NCHS | Patient location: National Center for Health Statistics (NCHS) urban-rural classification scheme for U.S. counties: (1) "Central" counties of metro areas of >=1 million population, (2) "Fringe" counties of metro areas of >=1 million population, (3) Counties in metro areas of 250,000-999,999 population, (4) Counties in metro areas of 50,000-249,999 population, (5) Micropolitan counties, (6) Not metropolitan or micropolitan counties | |
| ZIPINC_QRTL | Median household income quartiles for patient's ZIP Code. For 2013, the median income quartiles are defined as: (1) $1 - $37,999; (2) $38,000 - $47,999; (3) $48,000 - $63,999; and (4) $64,000 or more. | |
| Readmission Specific | DMONTH | Discharge month coded from (1) January to (12) December |
| NRD_DAYSTOEVENT | Count from "start date" to admission date coded differently for each value of NRD_VisitLink | |
| NRD_VISITLINK | Patient linkage number specific to the NRD and not linkable to any other HCUP or external databases | |
| REHABTRANSFER | A combined record involving transfer to rehabilitation, evaluation, or other aftercare: (1) yes, (0) no | |
| RESIDENT | Identifies patient as a resident of the State in which he or she received hospital care: (1) resident, (0) nonresident | |
| SAMEDAYEVENT | Identifies records that were combined from transfer or same-day stay pairs of records: (0) not a combined transfer or other same-day stay record, (1) combined transfer involving two discharges from different hospitals, (2) combined same-day stay involving two discharges at different hospitals, (3) combined same-day stay involving two discharges at the same hospital, (4) combined same-day stay involving three or more discharges at same or different hospitals | |
| Resource Use | LOS | Length of stay, edited |
| TOTCHG | Total charges, edited | |
| Weighting | DISCWT | NRD discharge weight to be used for calculating national estimates |
| NRD_STRATUM | NRD stratum for post-stratification based on geographic region, urban/rural location, teaching status, size of hospital based on number of beds, and control/ownership. For the confidentiality of hospitals and States, the NRD_STARTUM variable was randomly assigned into sequential numbers starting at 1. |
Table D.2. Data Elements in the NRD Severity File
| Category | Data Element Name | Description |
|---|---|---|
| 3M APR-DRG | APRDRG | 3M All Patient Refined DRG |
| APRDRG_Risk_Mortality | 3M All Patient Refined DRG: Risk of Mortality Subclass: (0) No class specified, (1) Minor likelihood of dying, (2) Moderate likelihood of dying, (3) Major likelihood of dying, (4) Extreme likelihood of dying | |
| APRDRG_Severity | 3M All Patient Refined DRG: Severity of Illness Subclass: (0) No class specified, (1) Minor loss of function (includes cases with no comorbidity or complications), (2) Moderate loss of function, (3) Major loss of function, (4) Extreme loss of function | |
| AHRQ Comorbidity Measures | CM_AIDS | AHRQ comorbidity measure - Acquired immune deficiency syndrome: (1) comorbidity present, (0) comorbidity not present |
| CM_ALCOHOL | AHRQ comorbidity measure - Alcohol abuse: (1) comorbidity present, (0) comorbidity not present | |
| CM_ANEMDEF | AHRQ comorbidity measure - Deficiency anemias: (1) comorbidity present, (0) comorbidity not present | |
| CM_ARTH | AHRQ comorbidity measure - Rheumatoid arthritis/collagen vascular diseases: (1) comorbidity present, (0) comorbidity not present | |
| CM_BLDLOSS | AHRQ comorbidity measure - Chronic blood loss anemia: (1) comorbidity present, (0) comorbidity not present | |
| CM_CHF | AHRQ comorbidity measure - Congestive heart failure: (1) comorbidity present, (0) comorbidity not present | |
| CM_CHRNLUNG | AHRQ comorbidity measure - Chronic pulmonary disease: (1) comorbidity present, (0) comorbidity not present | |
| CM_COAG | AHRQ comorbidity measure - Coagulopath: (1) comorbidity present, (0) comorbidity not present | |
| CM_DEPRESS | AHRQ comorbidity measure - Depression: (1) comorbidity present, (0) comorbidity not present | |
| CM_DM | AHRQ comorbidity measure - Diabetes, uncomplicated: (1) comorbidity present, (0) comorbidity not present | |
| CM_DMCX | AHRQ comorbidity measure - Diabetes with chronic complications: (1) comorbidity present, (0) comorbidity not present | |
| CM_DRUG | AHRQ comorbidity measure - Drug abuse: (1) comorbidity present, (0) comorbidity not present | |
| CM_HTN_C | AHRQ comorbidity measure - Hypertension, uncomplicated and complicated: (1) comorbidity present, (0) comorbidity not present | |
| CM_HYPOTHY | AHRQ comorbidity measure - Hypothyroidism: (1) comorbidity present, (0) comorbidity not present | |
| CM_LIVER | AHRQ comorbidity measure - Liver disease: (1) comorbidity present, (0) comorbidity not present | |
| CM_LYMPH | AHRQ comorbidity measure - Lymphoma: (1) comorbidity present, (0) comorbidity not present | |
| CM_LYTES | AHRQ comorbidity measure - Fluid and electrolyte disorders: (1) comorbidity present, (0) comorbidity not present | |
| CM_METS | AHRQ comorbidity measure - Metastatic cancer: (1) comorbidity present, (0) comorbidity not present | |
| CM_NEURO | AHRQ comorbidity measure - Other neurological disorders: (1) comorbidity present, (0) comorbidity not present | |
| CM_OBESE | AHRQ comorbidity measure - Obesity: (1) comorbidity present, (0) comorbidity not present | |
| CM_PARA | AHRQ comorbidity measure - Paralysis: (1) comorbidity present, (0) comorbidity not present | |
| CM_PERIVASC | AHRQ comorbidity measure - Peripheral vascular disorders: (1) comorbidity present, (0) comorbidity not present | |
| CM_PSYCH | AHRQ comorbidity measure - Psychoses: (1) comorbidity present, (0) comorbidity not present | |
| CM_PULMCIRC | AHRQ comorbidity measure - Pulmonary circulation disorders: (1) comorbidity present, (0) comorbidity not present | |
| CM_RENLFAIL | AHRQ comorbidity measure - Renal failure: (1) comorbidity present, (0) comorbidity not present | |
| CM_TUMOR | AHRQ comorbidity measure - Solid tumor without metastasis: (1) comorbidity present, (0) comorbidity not present | |
| CM_ULCER | AHRQ comorbidity measure - Peptic ulcer disease excluding bleeding: (1) comorbidity present, (0) comorbidity not present | |
| CM_VALVE | AHRQ comorbidity measure - Valvular disease: (1) comorbidity present, (0) comorbidity not present | |
| CM_WGHTLOSS | AHRQ comorbidity measure - Weight loss: (1) comorbidity present, (0) comorbidity not present | |
| NRD Identifiers | HOSP_NRD | NRD hospital identifier specific to the NRD and is not linkable to any other HCUP or external databases |
| KEY_NRD | NRD record identifier specific to the NRD and not linkable to any other HCUP or external databases |
Table D.3. Data Elements in the NRD Diagnosis and Procedure Groups File
| Category | Data Element Name | Description |
|---|---|---|
| Clinical Information | CHRON1-CHRON25 | Chronic condition indicator for all diagnoses: (0) non-chronic condition, (1) chronic condition |
| CHRONB1-CHRONB25 | Chronic Condition Indicators - body system for all diagnoses | |
| DXMCCS1 | Multi-level CCS category for principal diagnosis | |
| E_MCCS1 | Multi-level CCS category for first-listed E code | |
| PCLASS1-PCLASS15 | Procedure class for all ICD-9-CM procedures: (1) Minor Diagnostic, (2) Minor Therapeutic, (3) Major Diagnostic, (4) Major Therapeutic | |
| PRMCCS1 | Multi-level CCS category for first-listed procedure | |
| NRD Identifiers | HOSP_NRD | NRD hospital identifier specific to the NRD and is not linkable to any other HCUP or external databases |
| KEY_NRD | NRD record identifier specific to the NRD and not linkable to any other HCUP or external databases |
Table D.4. Data Elements in the NRD Hospital File
| Category | Data Element Name | Description |
|---|---|---|
| Admission/ Discharge | YEAR | Discharge year |
| Hospital Information | H_CONTROL | Control/ownership of hospital: (1) government, nonfederal [public], (2) private, not-for-profit [voluntary], (3) private, investor-owned [proprietary] |
| HOSP_BEDSIZE | Size of hospital based on the number of beds: (1) small, (2) medium, (3) large. The categories are defined using region of the U.S., the urban-rural designation of the hospital, in addition to the teaching status. | |
| HOSP_UR_TEACH | Teaching status of hospital: (0) metropolitan non-teaching, (1) metropolitan teaching, (2) non-metropolitan | |
| HOSP_URCAT4 | Hospital urban-rural location: (1) large metropolitan areas with at least 1 million residents, (2) small metropolitan areas with less than 1 million residents, (3) micropolitan areas, (4) not metropolitan or micropolitan, (8) metropolitan, collapsed category of large and small metropolitan, (9) non-metropolitan, collapsed category of micropolitan and rural | |
| NRD_STRATUM | NRD stratum for post-stratification based on geographic region, urban/rural location, teaching status, bed size, and control. Values range from 1 to 89. Region is not identified. | |
| NRD Identifiers | HOSP_NRD | NRD hospital identifier specific to the NRD and is not linkable to any other HCUP or external databases |
| Weighting | N_DISC_U | Number of discharges in the tart universe in the stratum |
| N_HOSP_U | Number of hospitals in the target universe in the stratum | |
| S_DISC_U | Number of NRD discharges in the stratum | |
| S_HOSP_U | Number of NRD hospitals in the stratum | |
| TOTAL_DISC | Total number of discharges for this hospital in the NRD |
APPENDIX E: EVALUATION OF THE DIFFERENCE IN READMISSION RATES CAUSED BY HCUP PATIENT LINKAGE NUMBERS BEING SPECIFIC TO A STATE
The HCUP revisit variables (NRD_VisitLink and NRD_DaysToEvent) that can be used to track patients across hospitalizations were created from patient linkage numbers provided by the HCUP Partners. These identifiers can only track patients across hospitals in a single State. Consider the following two illustrative examples:
The HCUP revisit variables are specific to tracking patients hospitalized in a State, regardless of whether the patient is a resident of the State. This limitation in the HCUP revisit variables causes the readmission rates to be artificially low. Consider the following example:
The true readmission rate across these two patients is 0.40 (0.40 = 2 readmissions / 5 index events). Using only California data, the readmission rate is 0.20 (0.20 = 1 readmissions / 5 index events). Using California and Florida data combined, the readmission rate is 0.17 (0.17 = 1 readmissions / 6 index events; there are 6 index events because the readmission for patient B in Florida gets counted as an index because it cannot be tied to the hospitalization in California).
We used the 2011 Medicare Standard Analytic File (SAF) to examine the impact on readmission rates caused by having State-specific patient linkage numbers. The SAF include patient linkage numbers that follow Medicare Fee-For-Service (FFS) patients across States; therefore, they do not have the same limitations as the HCUP data. We calculated condition-specific readmission rates in two ways. The index event for both readmission rates was allowed to include resident and nonresident discharges, similar to the NRD. The index events were grouped by the AHRQ Clinical Classifications Software (CCS) category for the principal diagnosis. For the first set of readmission rates, we required that a 30-day readmission for any cause occur in the same State as the index event, similar to the NRD. For the second set of readmission rates, we allowed the 30-day readmission for any cause to occur in any State. We limited the comparison of readmission rates to CCS categories with at least 100 index events. There were 247 CCS categories and the number of index events ranged from 101 to 468,709.
The analysis of readmission rates using the 2011 Medicare FFS data demonstrated that condition-specific readmission rates were higher if a patient could be tracked across all States, but that the percentage increase was less than 5 percent for most of the CCS categories. This analysis was limited in that it focused on the Medicare FFS population. The Medicare population accounted for 40 percent of all inpatient discharges in 2011,1 and previous research indicates that this population has higher readmission rates than discharges for other payers.2 Conditions often associated with younger adults, such as pregnancy, were included in the Medicare estimates because 20 percent of Medicare discharges are under the age of 65.3 Medicare patients under the age of 65 include people who are disabled or who have been diagnosed with end-stage renal disease or amyotrophic lateral sclerosis (ALS). Given the volume and severity of illness for Medicare patients, the estimates for the increase in the condition-specific readmission rates using the Medicare data provided a reasonable upper bound on the impact. The following three tables provide more detail on specific changes in readmission rates.
Table E.1 lists the percentage increase between the two types of readmission rates for the 20 conditions with the largest number of index events. These 20 conditions represent 54 percent of the index events in the analysis. The increase in the readmission rates when we capture readmission in other States ranged from 1.9 percent for urinary tract infections to 3.7 percent for chest pain.
Table E.1. Ten Conditions with the Largest Number of Index Events, Restricting Readmissions to Within State and Across All States
| Clinical Classifications Software Principal Diagnosis Category for the Index Event | Number of Index Events | 30-Day Readmission Rate per 100 Index Events | Percentage Increase in the Readmission Rates When Patients Were Followed Across States | |
|---|---|---|---|---|
| Readmissions Limited to the Same State as the Index Event | Readmissions Considered from Any State | |||
| 108: Congestive heart failure; nonhypertensive | 468,709 | 25.05 | 25.70 | 2.6 |
| 122: Pneumonia (except that caused by tuberculosis or sexually transmitted disease) | 443,388 | 18.25 | 18.69 | 2.4 |
| 2: Septicemia (except in labor) | 405,898 | 21.42 | 21.87 | 2.1 |
| 203: Osteoarthritis | 363,295 | 5.57 | 5.70 | 2.2 |
| 127: Chronic obstructive pulmonary disease and bronchiectasis | 356,562 | 21.99 | 22.40 | 1.9 |
| 106: Cardiac dysrhythmias | 345,225 | 16.73 | 17.27 | 3.3 |
| 159: Urinary tract infections | 277,674 | 18.31 | 18.66 | 1.9 |
| 237: Complication of device; implant or graft | 274,669 | 21.23 | 21.83 | 2.8 |
| 109: Acute cerebrovascular disease | 232,510 | 14.73 | 15.23 | 3.4 |
| 157: Acute and unspecified renal failure | 228,134 | 22.04 | 22.60 | 2.5 |
| 101: Coronary atherosclerosis and other heart disease | 220,481 | 15.44 | 15.99 | 3.5 |
| 100: Acute myocardial infarction | 205,949 | 19.66 | 20.41 | 3.8 |
| 55: Fluid and electrolyte disorders | 192,425 | 20.33 | 20.81 | 2.4 |
| 205: Spondylosis; intervertebral disc disorders; other back problems | 178,614 | 10.62 | 10.93 | 2.9 |
| 197: Skin and subcutaneous tissue infections | 178,097 | 16.09 | 16.45 | 2.2 |
| 226: Fracture of neck of femur (hip) | 172,566 | 13.89 | 14.19 | 2.2 |
| 50: Diabetes mellitus with complications | 159,421 | 23.81 | 24.31 | 2.1 |
| 238: Complications of surgical procedures or medical care | 155,584 | 20.50 | 21.08 | 2.9 |
| 153: Gastrointestinal hemorrhage | 155,410 | 18.75 | 19.27 | 2.8 |
| 102: Nonspecific chest pain | 148,512 | 13.50 | 14.00 | 3.7 |
Table E.2 lists the 10 conditions with the largest percentage differences between the two types of readmission rates. We expect the readmission rates using discharges across all States to be higher than the readmissions restricted to the same State as the index event. Only three CCS categories had an increase of more than 10 percent in the two readmission rates, and these CCS categories had a very small numbers of index events. The other seven CCS categories had a percentage increase between 6.6 percent and 7.9 percent and also had relatively small numbers of index events.
Table E.2. Ten Conditions with the Largest Difference in Readmission Rates, Restricting Readmissions to Within State and Across All States
| Clinical Classifications Software Principal Diagnosis Category for the Index Event | Number of Index Events | 30-Day Readmission Rate per 100 Index Events | Percentage Increase in the Readmission Rates When Patients Were Followed Across States | |
|---|---|---|---|---|
| Readmissions Limited to the Same State as the Index Event | Readmissions Considered from Any State | |||
| 188: Fetopelvic disproportion; obstruction | 153 | 4.58 | 5.23 | 14.3 |
| 20: Cancer; other respiratory and intrathoracic | 952 | 17.65 | 19.85 | 12.5 |
| 655: Mental disorders usually diagnosed in infancy, childhood, or adolescence | 115 | 15.65 | 17.39 | 11.1 |
| 227: Spinal cord injury | 2,420 | 17.69 | 19.09 | 7.9 |
| 185: Prolonged pregnancy | 665 | 2.11 | 2.26 | 7.2 |
| 77: Encephalitis (except that caused by tuberculosis or sexually transmitted disease) | 1,945 | 17.22 | 18.46 | 7.2 |
| 240: Burns | 4,110 | 16.40 | 17.54 | 7.0 |
| 213: Cardiac and circulatory congenital anomalies | 2,502 | 13.75 | 14.71 | 7.0 |
| 670: Miscellaneous disorders | 3,592 | 13.34 | 14.23 | 6.7 |
| 96: Heart valve disorders | 47,909 | 21.73 | 23.16 | 6.6 |
Table E.3 has the complete listing of readmissions rates for the 247 CCS categories. The majority of CCS categories (221 of the 247, 89.5 percent) had a percentage increase between the two types of readmission rates of less than 5 percent.
Table E.3. Readmission Rates Restricting Readmissions to Within State and Across All States, Medicare Standard Analytic File, 2011
| Clinical Classifications Software Principal Diagnosis Category for the Index Event | Number of Index Events | 30-Day Readmission Rate per 100 Index Events | Percentage Increase in the Readmission Rates When Patients Were Followed Across States | |
|---|---|---|---|---|
| Readmissions Limited to the Same State as the Index Event | Readmissions Considered from Any State | |||
| 1: Tuberculosis | 955 | 21.99 | 22.72 | 3.3 |
| 2: Septicemia (except in labor) | 405,898 | 21.42 | 31.87 | 2.1 |
| 3: Bacterial infection; unspecified site | 1,625 | 18.22 | 18.71 | 2.7 |
| 4: Mycoses | 10,755 | 27.56 | 28.10 | 2.0 |
| 5: HIV infection | 7,495 | 26.99 | 27.66 | 2.5 |
| 6: Hepatitis | 10,121 | 35.23 | 36.29 | 3.0 |
| 7: Viral infection | 14,033 | 16.01 | 16.46 | 2.8 |
| 8: Other infections; including parasitic | 4,117 | 13.46 | 14.09 | 4.7 |
| 9: Sexually transmitted infections (not HIV or hepatitis) | 699 | 14.16 | 14.45 | 2.0 |
| 10: Immunizations and screening for infectious disease | 149 | 14.09 | 14.09 | 60.0 |
| 11: Cancer of head and neck | 8,915 | 20.43 | 21.40 | 0.0 |
| 12: Cancer of esophagus | 3,956 | 26.69 | 27.68 | 3.7 |
| 13: Cancer of stomach | 7,171 | 23.79 | 24.68 | 3.8 |
| 14: Cancer of colon | 37,987 | 17.16 | 17.53 | 2.2 |
| 15: Cancer of rectum and anus | 12,496 | 21.26 | 21.68 | 2.0 |
| 16: Cancer of liver and intrahepatic bile duct | 5,935 | 24.08 | 25.14 | 4.4 |
| 17: Cancer of pancreas | 12,641 | 24.41 | 25.74 | 5.5 |
| 18: Cancer of other GI organs; peritoneum | 7,043 | 24.00 | 25.27 | 5.3 |
| 19: Cancer of bronchus; lung | 51,885 | 20.46 | 21.12 | 3.2 |
| 20: Cancer; other respiratory and intrathoracic | 952 | 17.65 | 19.85 | 12.5 |
| 21: Cancer of bone and connective tissue | 3,007 | 20.02 | 20.82 | 4.0 |
| 22: Melanomas of skin | 989 | 13.25 | 13.65 | 3.0 |
| 23: Other non-epithelial cancer of skin | 2,483 | 15.99 | 16.31 | 2.0 |
| 24: Cancer of breast | 18,788 | 9.27 | 9.41 | 1.5 |
| 25: Cancer of uterus | 10,304 | 11.81 | 12.35 | 4.5 |
| 26: Cancer of cervix | 1,616 | 20.92 | 21.78 | 4.1 |
| 27: Cancer of ovary | 6,450 | 21.83 | 22.71 | 4.0 |
| 28: Cancer of other female genital organs | 2,155 | 15.82 | 16.15 | 2.0 |
| 29: Cancer of prostate | 24,323 | 6.93 | 7.13 | 3.0 |
| 31: Cancer of other male genital organs | 308 | 22.73 | 23.38 | 2.9 |
| 32: Cancer of bladder | 14,780 | 22.18 | 23.03 | 3.8 |
| 33: Cancer of kidney and renal pelvis | 14,717 | 13.23 | 18.84 | 4.6 |
| 34: Cancer of other urinary organs | 1,716 | 16.14 | 16.96 | 5.1 |
| 35: Cancer of brain and nervous system | 7,670 | 20.38 | 21.51 | 5.6 |
| 36: Cancer of thyroid | 3,775 | 9.35 | 9.48 | 1.4 |
| 37: Hodgkin's disease | 584 | 32.36 | 33.39 | 3.2 |
| 38: Non-Hodgkin's lymphoma | 12,102 | 34.35 | 35.44 | 3.2 |
| 39: Leukemias | 8,871 | 33.62 | 34.86 | 3.7 |
| 40: Multiple myeloma | 5,748 | 28.97 | 29.91 | 3.2 |
| 41: Cancer; other and unspecified primary | 1,514 | 16.84 | 17.90 | 6.3 |
| 42: Secondary malignancies | 65,482 | 23.08 | 23.97 | 3.8 |
| 43: Malignant neoplasm without specification of site | 2,667 | 24.03 | 24.82 | 3.3 |
| 44: Neoplasms of unspecified nature or uncertain behavior | 14,836 | 25.74 | 26.54 | 3.1 |
| 45: Maintenance chemotherapy; radiotherapy | 25,281 | 61.29 | 61.98 | 1.1 |
| 46: Benign neoplasm of uterus | 4,909 | 7.29 | 7.37 | 1.1 |
| 47: Other and unspecified benign neoplasm | 34,259 | 12.74 | 13.18 | 3.4 |
| 48: Thyroid disorders | 9,288 | 10.81 | 11.00 | 1.8 |
| 49: Diabetes mellitus without complication | 3,189 | 16.09 | 16.31 | 1.4 |
| 50: Diabetes mellitus with complications | 159,421 | 23.81 | 24.31 | 2.1 |
| 51: Other endocrine disorders | 23,041 | 20.09 | 20.52 | 2.1 |
| 52: Nutritional deficiencies | 4,402 | 24.49 | 24.97 | 1.9 |
| 53: Disorders of lipid metabolism | 147 | 14.29 | 14.29 | 0.0 |
| 54: Gout and other crystal arthropathies | 9,186 | 17.46 | 17.92 | 2.6 |
| 55: Fluid and electrolyte disorders | 192,425 | 20.33 | 20.81 | 2.4 |
| 56: Cystic fibrosis | 1,507 | 20.31 | 20.77 | 2.3 |
| 57: Immunity disorders | 306 | 28.76 | 30.07 | 4.5 |
| 58: Other nutritional; endocrine; and metabolic disorders | 33,388 | 16.81 | 17.25 | 2.6 |
| 59: Deficiency and other anemia | 92,656 | 23.19 | 23.74 | 2.4 |
| 60: Acute posthemorrhagic anemia | 16,095 | 21.09 | 21.66 | 2.7 |
| 61: Sickle cell anemia | 15,360 | 34.26 | 35.22 | 2.8 |
| 62: Coagulation and hemorrhagic disorders | 9,731 | 27.73 | 28.53 | 2.9 |
| 63: Diseases of white blood cells | 17,437 | 26.37 | 27.06 | 2.6 |
| 64: Other hematologic conditions | 1,256 | 22.05 | 22.93 | 4.0 |
| 76: Meningitis (except that caused by tuberculosis or sexually transmitted disease) | 3,339 | 16.17 | 17.07 | 5.6 |
| 77: Encephalitis (except that caused by tuberculosis or sexually transmitted disease) | 1,945 | 17.22 | 18.46 | 7.2 |
| 78: Other CNS infection and poliomyelitis | 2,052 | 22.86 | 23.64 | 3.4 |
| 79: Parkinson's disease | 6,647 | 13.25 | 13.44 | 1.4 |
| 80: Multiple sclerosis | 6,730 | 13.80 | 14.28 | 3.4 |
| 81: Other hereditary and degenerative nervous system conditions | 17,788 | 16.29 | 16.83 | 3.3 |
| 82: Paralysis | 1,732 | 17.21 | 17.55 | 2.0 |
| 83: Epilepsy; convulsions | 70,843 | 15.62 | 16.10 | 3.1 |
| 84: Headache; including migraine | 12,433 | 13.04 | 13.56 | 4.0 |
| 85: Coma; stupor; and brain damage | 7,730 | 16.96 | 17.50 | 3.2 |
| 87: Retinal detachments; defects; vascular occlusion; and retinopathy | 1,091 | 9.90 | 10.36 | 4.6 |
| 88: Glaucoma | 274 | 13.14 | 13.50 | 2.8 |
| 89: Blindness and vision defects | 1,943 | 12.30 | 13.02 | 5.9 |
| 90: Inflammation; infection of eye (except that caused by tuberculosis or sexually transmitted disease) | 3,104 | 13.24 | 13.85 | 4.6 |
| 91: Other eye disorders | 1,835 | 11.55 | 12.15 | 5.2 |
| 92: Otitis media and related conditions | 1,410 | 11.49 | 12.13 | 5.6 |
| 93: Conditions associated with dizziness or vertigo | 29,165 | 8.27 | 8.53 | 3.2 |
| 94: Other ear and sense organ disorders | 1,672 | 13.22 | 13.64 | 3.2 |
| 95: Other nervous system disorders | 88,536 | 18.37 | 18.94 | 3.1 |
| 96: Heart valve disorders | 47,909 | 21.73 | 23.16 | 6.6 |
| 97: Peri-; endo-; and myocarditis; cardiomyopathy (except that caused by tuberculosis or sexually transmitted disease) | 19,317 | 21.89 | 22.71 | 3.8 |
| 98: Essential hypertension | 21,797 | 11.97 | 12.30 | 2.8 |
| 99: Hypertension with complications and secondary hypertension | 101,943 | 24.08 | 24.58 | 2.1 |
| 100: Acute myocardial infarction | 205,949 | 19.66 | 20.41 | 3.8 |
| 101: Coronary atherosclerosis and other heart disease | 220,481 | 15.44 | 15.99 | 3.5 |
| 102: Nonspecific chest pain | 148,512 | 13.50 | 14.00 | 3.7 |
| 103: Pulmonary heart disease | 67,061 | 16.71 | 17.20 | 2.9 |
| 104: Other and ill-defined heart disease | 3,266 | 13.38 | 14.15 | 5.7 |
| 105: Conduction disorders | 29,215 | 11.42 | 11.86 | 3.9 |
| 106: Cardiac dysrhythmias | 345,225 | 16.73 | 17.27 | 3.3 |
| 107: Cardiac arrest and ventricular fibrillation | 3,236 | 18.88 | 19.96 | 5.7 |
| 108: Congestive heart failure; nonhypertensive | 468,709 | 25.05 | 25.70 | 2.6 |
| 109: Acute cerebrovascular disease | 232,510 | 14.73 | 15.23 | 3.4 |
| 110: Occlusion or stenosis of precerebral arteries | 59,225 | 10.67 | 10.97 | 2.8 |
| 111: Other and ill-defined cerebrovascular disease | 7,927 | 11.30 | 11.86 | 4.9 |
| 112: Transient cerebral ischemia | 80,396 | 11.31 | 11.66 | 3.1 |
| 113: Late effects of cerebrovascular disease | 8,073 | 15.88 | 16.31 | 2.7 |
| 114: Peripheral and visceral atherosclerosis | 77,210 | 19.15 | 19.65 | 2.6 |
| 115: Aortic; peripheral; and visceral artery aneurysms | 35,223 | 15.16 | 15.95 | 5.2 |
| 116: Aortic and peripheral arterial embolism or thrombosis | 11,656 | 22.50 | 23.07 | 2.6 |
| 117: Other circulatory disease | 61,972 | 17.52 | 17.98 | 2.6 |
| 118: Phlebitis; thrombophlebitis and thromboembolism | 67,493 | 16.60 | 17.07 | 2.8 |
| 119: Varicose veins of lower extremity | 1,061 | 16.21 | 16.68 | 2.9 |
| 120: Hemorrhoids | 12,573 | 17.41 | 17.74 | 1.9 |
| 121: Other diseases of veins and lymphatics | 9,833 | 20.34 | 21.06 | 3.5 |
| 122: Pneumonia (except that caused by tuberculosis or sexually transmitted disease) | 443,388 | 18.25 | 18.69 | 2.4 |
| 123: Influenza | 11,775 | 11.77 | 12.03 | 2.2 |
| 124: Acute and chronic tonsillitis | 809 | 7.17 | 7.54 | 5.2 |
| 125: Acute bronchitis | 26,446 | 13.00 | 13.32 | 2.4 |
| 126: Other upper respiratory infections | 9,847 | 13.01 | 13.48 | 3.6 |
| 127: Chronic obstructive pulmonary disease and bronchiectasis | 356,562 | 21.99 | 22.40 | 1.9 |
| 128: Asthma | 85,354 | 18.61 | 18.99 | 2.0 |
| 129: Aspiration pneumonitis; food/vomitus | 95,063 | 20.93 | 21.22 | 1.4 |
| 130: Pleurisy; pneumothorax; pulmonary collapse | 37,794 | 25.02 | 25.81 | 3.1 |
| 131: Respiratory failure; insufficiency; arrest (adult) | 136,502 | 24.58 | 25.19 | 2.5 |
| 132: Lung disease due to external agents | 2,365 | 20.25 | 21.02 | 3.8 |
| 133: Other lower respiratory disease | 44,901 | 18.94 | 19.54 | 3.1 |
| 134: Other upper respiratory disease | 12,106 | 18.43 | 19.09 | 3.6 |
| 135: Intestinal infection | 86,677 | 22.59 | 23.04 | 2.0 |
| 136: Disorders of teeth and jaw | 3,436 | 12.34 | 12.75 | 3.3 |
| 137: Diseases of mouth; excluding dental | 5,261 | 14.94 | 15.32 | 2.5 |
| 138: Esophageal disorders | 41,912 | 16.29 | 16.70 | 2.5 |
| 139: Gastroduodenal ulcer (except hemorrhage) | 12,983 | 17.04 | 17.62 | 3.4 |
| 140: Gastritis and duodenitis | 34,213 | 19.14 | 19.57 | 2.2 |
| 141: Other disorders of stomach and duodenum | 26,484 | 24.64 | 25.32 | 2.8 |
| 142: Appendicitis and other appendiceal conditions | 18,638 | 9.55 | 9.84 | 3.0 |
| 143: Abdominal hernia | 62,904 | 13.38 | 13.74 | 2.6 |
| 144: Regional enteritis and ulcerative colitis | 16,244 | 23.15 | 24.02 | 3.7 |
| 145: Intestinal obstruction without hernia | 131,147 | 17.46 | 17.93 | 2.7 |
| 146: Diverticulosis and diverticulitis | 107,020 | 14.55 | 14.91 | 2.5 |
| 147: Anal and rectal conditions | 13,837 | 16.30 | 16.68 | 2.3 |
| 148: Peritonitis and intestinal abscess | 8,092 | 27.39 | 28.28 | 3.2 |
| 149: Biliary tract disease | 107,986 | 14.87 | 15.38 | 3.4 |
| 151: Other liver diseases | 36,907 | 32.88 | 34.08 | 3.7 |
| 152: Pancreatic disorders (not diabetes) | 66,802 | 19.80 | 20.58 | 4.0 |
| 153: Gastrointestinal hemorrhage | 155,410 | 18.75 | 19.27 | 2.8 |
| 154: Noninfectious gastroenteritis | 41,796 | 16.12 | 16.57 | 2.8 |
| 155: Other gastrointestinal disorders | 73,673 | 20.39 | 20.92 | 2.6 |
| 156: Nephritis; nephrosis; renal sclerosis | 1,488 | 25.00 | 25.54 | 2.2 |
| 157: Acute and unspecified renal failure | 228,134 | 22.04 | 22.60 | 2.5 |
| 158: Chronic kidney disease | 8,075 | 26.13 | 26.81 | 2.6 |
| 159: Urinary tract infections | 277,674 | 18.31 | 18.66 | 1.9 |
| 160: Calculus of urinary tract | 32,158 | 13.21 | 13.53 | 2.4 |
| 161: Other diseases of kidney and ureters | 12,302 | 19.05 | 19.64 | 3.1 |
| 162: Other diseases of bladder and urethra | 8,649 | 18.28 | 18.582 | 3.0 |
| 163: Genitourinary symptoms and ill-defined conditions | 15,706 | 20.16 | 20.69 | 2.6 |
| 164: Hyperplasia of prostate | 23,248 | 11.51 | 11.75 | 2.1 |
| 165: Inflammatory conditions of male genital organs | 6,873 | 13.90 | 14.22 | 2.3 |
| 166: Other male genital disorders | 2,842 | 15.94 | 16.50 | 3.5 |
| 167: Nonmalignant breast conditions | 2,997 | 13.38 | 13.65 | 2.0 |
| 168: Inflammatory diseases of female pelvic organs | 3,024 | 15.18 | 15.68 | 3.3 |
| 169: Endometriosis | 940 | 7.98 | 8.19 | 2.7 |
| 170: Prolapse of female genital organs | 25,446 | 3.53 | 3.66 | 3.7 |
| 171: Menstrual disorders | 3,085 | 9.17 | 9.37 | 2.1 |
| 172: Ovarian cyst | 2,420 | 10.29 | 10.50 | 2.0 |
| 173: Menopausal disorders | 2,132 | 12.48 | 12.81 | 2.6 |
| 175: Other female genital disorders | 7,410 | 11.86 | 12.27 | 3.4 |
| 177: Spontaneous abortion | 119 | 6.72 | 6.72 | 0.0 |
| 180: Ectopic pregnancy | 159 | 8.18 | 8.18 | 0.0 |
| 181: Other complications of pregnancy | 3,919 | 20.31 | 20.80 | 2.4 |
| 182: Hemorrhage during pregnancy; abruptio placenta; placenta previa | 266 | 17.67 | 18.80 | 26.4 |
| 183: Hypertension complicating pregnancy; childbirth and the puerperium | 1,544 | 16.78 | 16.90 | 0.8 |
| 184: Early or threatened labor | 1,068 | 23.69 | 24.06 | 1.6 |
| 185: Prolonged pregnancy | 665 | 2.11 | 2.26 | 7.2 |
| 186: Diabetes or abnormal glucose tolerance complicating pregnancy; childbirth; or the puerperium | 822 | 19.34 | 19.83 | 2.5 |
| 187: Malposition; malpresentation | 414 | 4.59 | 4.59 | 2.0 |
| 188: Fetopelvic disproportion; obstruction | 153 | 4.58 | 5.23 | 14.3 |
| 189: Previous C-section | 2,229 | 3.99 | 4.08 | 2.3 |
| 190: Fetal distress and abnormal forces of labor | 584 | 4.62 | 4.62 | 0.0 |
| 191: Polyhydramnios and other problems of amniotic cavity | 821 | 8.53 | 8.77 | 2.9 |
| 192: Umbilical cord complication | 421 | 1.43 | 1.43 | 0.0 |
| 193: OB-related trauma to perineum and vulva | 1,159 | 1.12 | 1.12 | 0.0 |
| 195: Other complications of birth; puerperium affecting management of mother | 2,716 | 8.51 | 8.62 | 1.3 |
| 196: Normal pregnancy and/or delivery | 450 | 1.56 | 1.56 | 0.0 |
| 197: Skin and subcutaneous tissue infections | 178,097 | 16.09 | 16.45 | 2.2 |
| 198: Other inflammatory condition of skin | 2,901 | 21.06 | 21.68 | 2.9 |
| 199: Chronic ulcer of skin | 25,685 | 21.13 | 21.62 | 2.3 |
| 200: Other skin disorders | 2,589 | 17.69 | 18.00 | 1.7 |
| 201: Infective arthritis and osteomyelitis (except that caused by tuberculosis or sexually transmitted disease) | 24,731 | 19.24 | 19.77 | 2.8 |
| 202: Rheumatoid arthritis and related disease | 5,487 | 12.96 | 13.30 | 2.7 |
| 203: Osteoarthritis | 363,295 | 5.57 | 5.70 | 2.2 |
| 204: Other non-traumatic joint disorders | 16,187 | 11.90 | 12.27 | 3.1 |
| 205: Spondylosis; intervertebral disc disorders; other back problems | 178,614 | 10.62 | 10.93 | 2.9 |
| 206: Osteoporosis | 146 | 21.23 | 21.92 | 3.2 |
| 207: Pathological fracture | 31,718 | 19.03 | 19.46 | 2.3 |
| 208: Acquired foot deformities | 1,705 | 7.57 | 7.68 | 1.5 |
| 209: Other acquired deformities | 17,245 | 9.92 | 10.22 | 3.0 |
| 210: Systemic lupus erythematosus and connective tissue disorders | 3,920 | 26.79 | 27.37 | 2.2 |
| 211: Other connective tissue disease | 47,933 | 13.59 | 14.00 | 3.0 |
| 212: Other bone disease and musculoskeletal deformities | 21,751 | 12.33 | 12.63 | 2.5 |
| 213: Cardiac and circulatory congenital anomalies | 2,502 | 13.75 | 14.71 | 7.0 |
| 214: Digestive congenital anomalies | 713 | 15.71 | 16.69 | 6.3 |
| 215: Genitourinary congenital anomalies | 1,201 | 16.24 | 16.57 | 2.1 |
| 216: Nervous system congenital anomalies | 397 | 17.88 | 18.39 | 2.8 |
| 217: Other congenital anomalies | 4,654 | 8.10 | 8.36 | 3.2 |
| 225: Joint disorders and dislocations; trauma-related | 6,439 | 11.14 | 11.34 | 1.8 |
| 226: Fracture of neck of femur (hip) | 172,566 | 13.89 | 14.19 | 2.2 |
| 227: Spinal cord injury | 2,420 | 17.69 | 19.09 | 7.9 |
| 228: Skull and face fractures | 7,091 | 11.90 | 12.38 | 4.0 |
| 229: Fracture of upper limb | 43,161 | 11.92 | 12.33 | 3.5 |
| 230: Fracture of lower limb | 61,816 | 13.59 | 13.96 | 2.7 |
| 231: Other fractures | 87,204 | 14.05 | 14.50 | 3.2 |
| 232: Sprains and strains | 9,057 | 11.52 | 11.91 | 3.4 |
| 233: Intracranial injury | 55,231 | 16.40 | 17.16 | 4.6 |
| 234: Crushing injury or internal injury | 13,358 | 15.29 | 15.98 | 4.5 |
| 235: Open wounds of head; neck; and trunk | 5,616 | 12.29 | 12.84 | 4.5 |
| 236: Open wounds of extremities | 5,507 | 13.78 | 14.11 | 2.4 |
| 237: Complication of device; implant or graft | 274,669 | 21.23 | 21.83 | 2.8 |
| 238: Complications of surgical procedures or medical care | 155,584 | 20.50 | 21.08 | 2.9 |
| 239: Superficial injury; contusion | 16,861 | 15.88 | 16.23 | 2.2 |
| 240: Burns | 4,110 | 16.40 | 17.54 | 7.0 |
| 241: Poisoning by psychotropic agents | 14,978 | 12.82 | 13.27 | 3.5 |
| 242: Poisoning by other medications and drugs | 27,934 | 15.48 | 15.99 | 3.3 |
| 243: Poisoning by nonmedicinal substances | 2,659 | 11.51 | 12.19 | 5.9 |
| 244: Other injuries and conditions due to external causes | 19,846 | 15.79 | 16.34 | 3.5 |
| 245: Syncope | 95,001 | 11.90 | 12.29 | 3.2 |
| 246: Fever of unknown origin | 17,447 | 19.54 | 20.14 | 3.1 |
| 247: Lymphadenitis | 1,297 | 18.50 | 19.20 | 3.8 |
| 248: Gangrene | 17,575 | 33.79 | 34.34 | 1.6 |
| 249: Shock | 554 | 23.11 | 24.19 | 4.7 |
| 250: Nausea and vomiting | 16,042 | 22.97 | 23.48 | 2.2 |
| 251: Abdominal pain | 36,919 | 20.14 | 20.89 | 3.7 |
| 252: Malaise and fatigue | 14,952 | 16.77 | 17.19 | 2.5 |
| 253: Allergic reactions | 5,477 | 15.45 | 15.94 | 3.2 |
| 254: Rehabilitation care; fitting of prostheses; and adjustment of devices | 1,558 | 11.04 | 11.10 | 0.6 |
| 256: Medical examination/evaluation | 101 | 22.77 | 23.76 | 4.3 |
| 257: Other aftercare | 1,889 | 14.08 | 14.45 | 2.6 |
| 258: Other screening for suspected conditions (not mental disorders or infectious disease) | 522 | 17.43 | 17.63 | 1.1 |
| 259: Residual codes; unclassified | 43,683 | 18.57 | 19.14 | 3.0 |
| 650: Adjustment disorders | 1,361 | 13.45 | 13.89 | 3.3 |
| 651: Anxiety disorders | 4,677 | 15.72 | 16.02 | 1.9 |
| 652: Attention-deficit | 245 | 19.59 | 20.00 | 2.1 |
| 653: Delirium | 37,650 | 14.28 | 14.72 | 3.1 |
| 654: Developmental disorders | 613 | 19.09 | 19.41 | 1.7 |
| 655: Disorders usually diagnosed in infancy | 115 | 15.65 | 17.39 | 11.1 |
| 656: Impulse control disorders | 417 | 17.03 | 17.51 | 2.8 |
| 657: Mood disorders | 45,648 | 18.81 | 19.38 | 3.0 |
| 658: Personality disorders | 431 | 25.52 | 26.45 | 3.6 |
| 659: Schizophrenia and other psychotic disorders | 47,114 | 22.04 | 22.37 | 1.5 |
| 660: Alcohol-related disorders | 30,228 | 20.72 | 21.24 | 2.5 |
| 661: Substance-related disorders | 32,424 | 18.68 | 19.28 | 3.2 |
| 662: Suicide and intentional self-inflicted injury | 240 | 16.67 | 17.50 | 5.0 |
| 663: Screening and history of mental health and substance abuse codes | 11,302 | 28.35 | 29.03 | 2.4 |
| 670: Miscellaneous disorders | 3,592 | 13.34 | 14.23 | 6.7 |
1 If the patient is transferred to an out-of-state hospital, the subsequent discharge would not be included in the NRD because the HCUP patient linkage numbers only can follow a patient within a State.
2 Although the text refers to using the discharge and admission date, in reality, we used the NRD data element NRD_DaysToEvent and LOS to identify the sequential order of inpatient records and whether they stopped and started on the same day.
3 Admission and discharge dates were not available because of patient confidentiality restrictions.
4 Total hospital cost must be added to the NRD using the HCUP supplemental Cost-to-Charge Ratio files.
5 Changes in the NIS Sampling and Weighting Strategy for 1998. ONLINE January 18, 2002. Available at http://www.hcup-us.ahrq.gov/db/nation/nis/reports/Changes_in_NIS_Design_1998.pdf. Accessed September 15, 2011.
6 HCUPnet query on the expected primary payer for the 2011 Nationwide Inpatient Sample. Accessed December 9, 2014.
7 Wier LM, Barrett ML, Steiner C, Jiang HJ. All-Cause Readmissions by Payer and Age, 2008. HCUP Statistical Brief No. 115. June 2011. Rockville, MD: Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb115.pdf.
8 HCUPnet query on the expected primary payer and age for the 2011Nationwide Inpatient Sample. Accessed December 9, 2014.
9 Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M. Methodological Issues when Studying Readmissions and Revisits Using Hospital Administrative Data. HCUP Methods Series Report No. 2011-01. Online March 9, 2011. Rockville, MD: U.S. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/methods/2011_01.pdf.
10 Carlson BL, Johnson AE, Cohen SB. An evaluation of the use of personal computers for variance estimation with complex survey data. J Off Statistics. 1993;9(4):795-814.
11 If the patient is transferred to an out-of-State hospital, the subsequent discharge would not be included in the NRD because the HCUP patient linkage numbers only can follow a patient within a State.
12 Although the text refers to using the discharge and admission date, in reality, we used the NRD data element NRD_DaysToEvent and LOS to identify the sequential order of inpatient records and whether they stopped and started on the same day.
Appendix E Footnotes
1 HCUPnet query on the expected primary payer for the 2011 Nationwide Inpatient Sample. Accessed December 9, 2014.
2 Wier LM, Barrett ML, Steiner C, Jiang HJ. All-Cause Readmissions by Payer and Age, 2008. HCUP Statistical Brief #115. June 2011. Rockville, MD: Agency for Healthcare Research and Quality.
3 HCUPnet query on the expected primary payer and age for the 2011 Nationwide Inpatient Sample. Accessed December 9, 2014.
| Internet Citation: 2013 Introduction to the NRD. Healthcare Cost and Utilization Project (HCUP). December 2015. Agency for Healthcare Research and Quality, Rockville, MD. hcup-us.ahrq.gov/db/nation/nrd/NRD_Introduction_2013.jsp. |
| Are you having problems viewing or printing pages on this website? |
| If you have comments, suggestions, and/or questions, please contact hcup@ahrq.gov. |
| If you are experiencing issues related to Section 508 accessibility of information on this website, please contact hcup@ahrq.gov. |
| Privacy Notice, Viewers & Players |
| Last modified 12/21/15 |